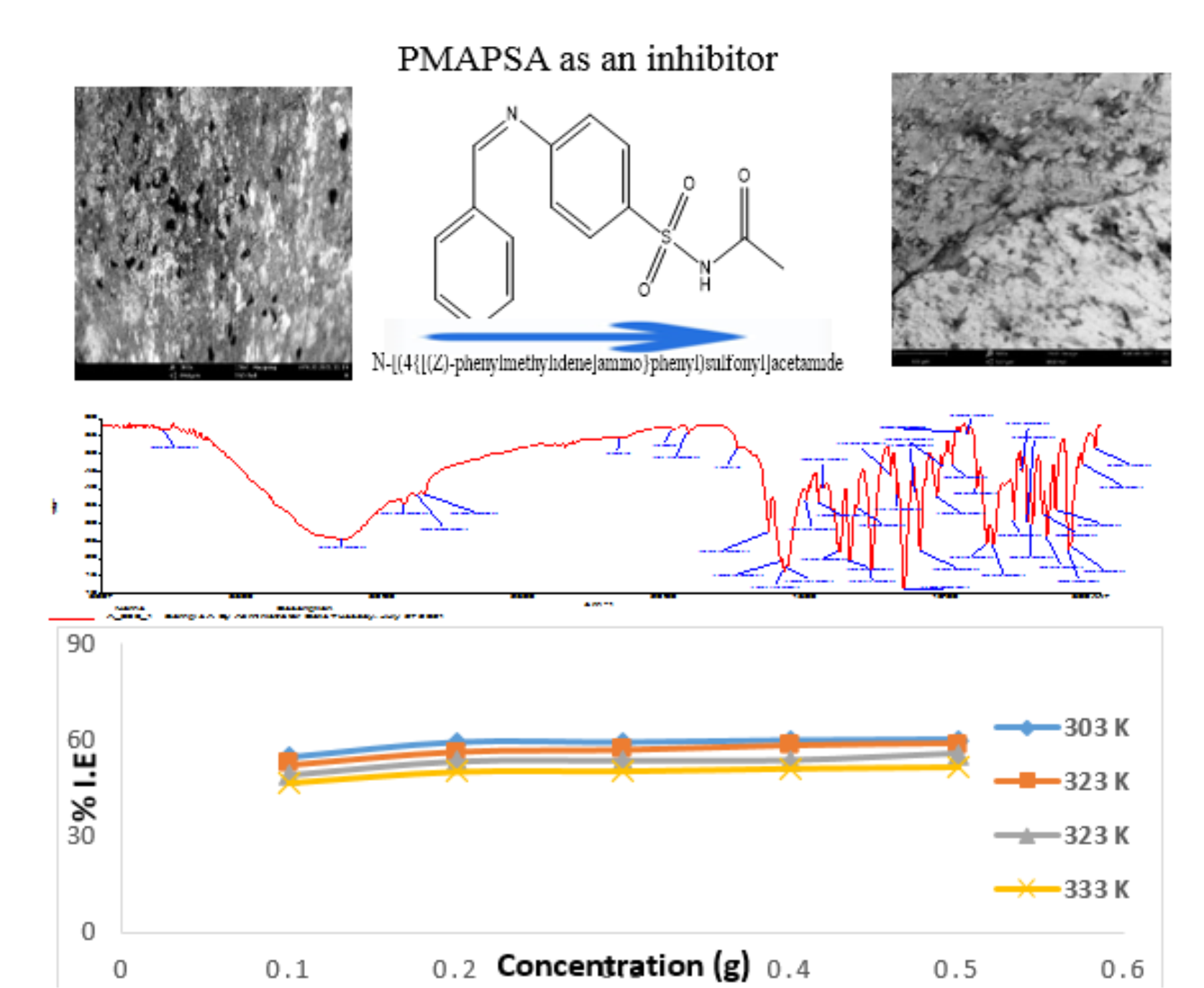
Adsorption and thermodynamic study of the inhibition of corrosion of zinc in HCl medium using N-[(4{[(Z)-phenylmethylidene]amino}phenyl)sulfonyl] acetamide
Keywords:
Corrosion inhibition; inhibitor; adsorption, thermodynamicsAbstract
The inhibition and adsorption potential of Schiff’s base, N-[(4{[(Z)-phenyl methylidene]amino}phenyl)sulfonyl]acetamide (PMAPSA) for zinc corrosion in HCl solution at different temperatures were studied using gravimetric method. The PMAPSA, uninhibited and inhibited zinc samples were characterized using the Fourier transform infra-red spectrum, X-ray diffraction and scanning electron microscope (SEM). The inhibitive effect of PMAPSA depend on the immersion period, PMAPSA concentration and temperature. The study revealed that the higher the PMAPSA concentration the lower the weight loss. The inhibitor, PMAPSA was found to exhibit a maximum inhibitive efficiency of 65.1 % at 0.5 g of PMAPSA and at 303 K. The activation energy of the inhibited corrosion reaction ranged from 9.72 kJ mol-1 to 11.24 kJ mol-1 with 10.39 kJ mol-1 (average) which is more than the 4.49 kJmol-1 obtained for the uninhibited. Thermodynamic assessment of the corrosion process revealed that the PMAPSA adsorption on the zinc surface is endothermic and spontaneous. The uptake characteristic of PMAPSA was best described by Langmuir isotherm and this further buttressed the conclusion that the mode of adsorption of PMAPSA may have followed chemisorption mechanism.