Kinetic and Modeling of Anionic Dye Adsorption onto Acid modified Ihiala Clays: ANN, ANFIS and RSM comparative analysis
Keywords:
adsorption; kinetics; modeling; thermodynamics; artificial neural networksAbstract
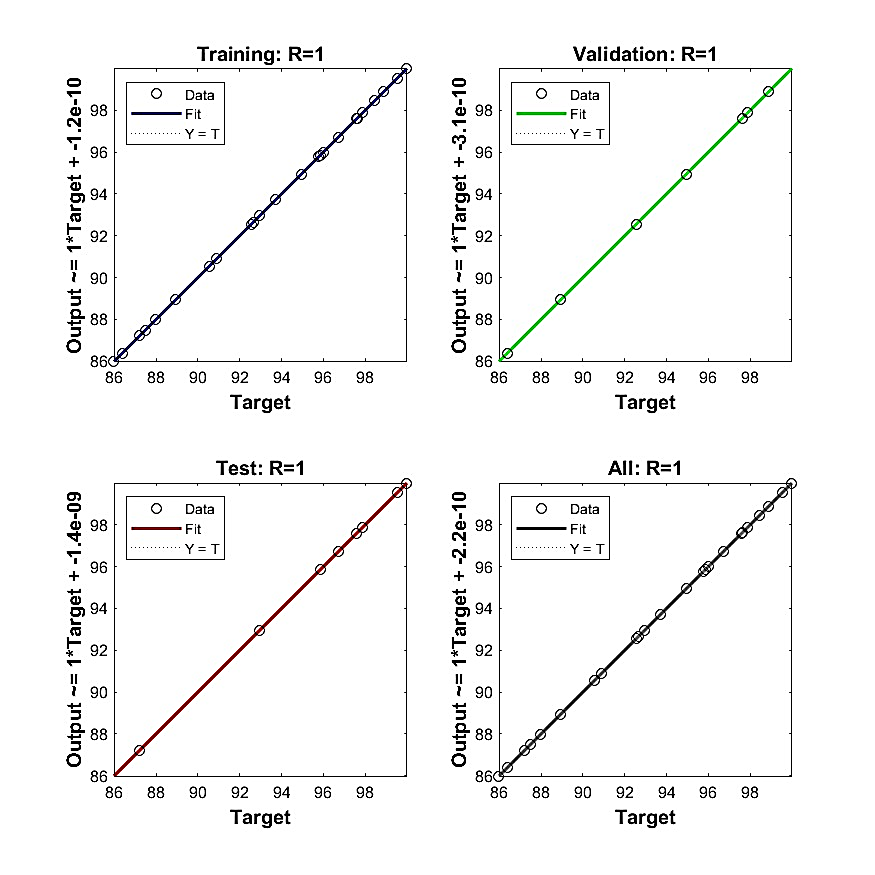
Congo red (CR) dye is a harmful dye of environmental concern due to its complex molecular structure and artificial origin, which make it non-biodegradable. The research focused on using the physico-chemical method to remove CR pollutants (color, dissolved salt (EC), total dissolved solids (TDS), and chemical oxygen demand (COD)) from effluent by utilizing the adsorptive qualities of acid-modified Ihiala clay (HIC). The batch system was applied to evaluate the effect of process-independent variables on the adsorption process. The mechanism of adsorption was investigated using intra-particle diffusion, liquid film, the Bangham model, and the Boyd model. The thermodynamic properties ∆S, ∆H, ∆G, and ∆Ea were determined. The optimum removal efficiency of CR was predicted using the ANN, ANFIS, and RSM models. The activation resulted in an increase in surface area. Maximum color removal of 99.3% was observed at pH 2, an adsorbent dosage of 1 g, an adsorbent particle size of 75 µm, an initial dye concentration of 100 mg/l, a contact time of 120 min, and a temperature of 323 k. A maximum adsorption capacity of 86.70 mg/g was obtained. The liquid film diffusion was the major rate-limiting step. Thermodynamic results suggested an endothermic, favorable, spontaneous, and physical adsorption process. The RSM model is statistically more significant than the ANFIS and ANN models. A maximum desorption capacity of 90.3% was achieved after four cycles. The obtained results confirm HIC as a reliable, cost-effective adsorbent for CR pollutants removal from effluents.