Kinetic Analysis and Adsorptive Efficiency in Ciprofloxacin Removal from Pharmaceutical Waste-water using an Effective Adsorbent
Keywords:
Palm tree trunk; Adsorption; Ciprofloxacin; Kinetics models; Isotherms modelsAbstract
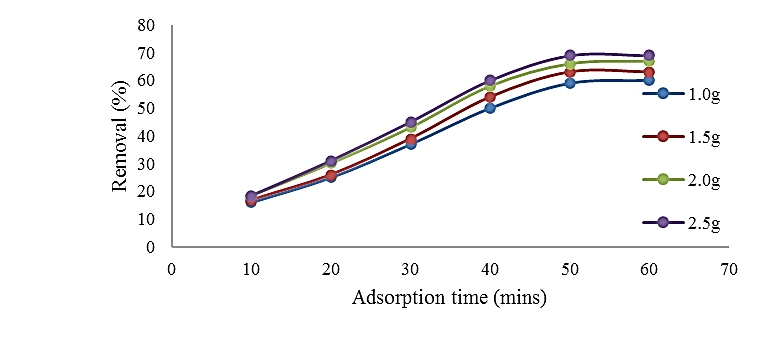
This work exploits palm tree trunk (PTT), an agricultural byproduct, as an efficient adsorbent to remove Ciprofloxacin (CIP) chemicals from pharmaceutical wastewater. The batch adsorption procedure was used to examine the effects of parameters on the adsorption rate, including contact duration, adsorbent dosage, temperature, concentration, and pH. The sorption potential of palm tree trunks was investigated further using studies on adsorption kinetics, thermodynamics, and isotherms. At an ideal pH of 4, the percentage of CIP removed by PTT was 90.94 %. Ciprofloxacin adsorption on the same adsorbent improved as the concentration of ciprofloxacin in wastewater reduced, the solution pH increased to 7, and the solution temperature rose to 50° C. The Freundlich, Temkin, and Langmuir isotherm models examined the equilibrium data. The maximum adsorption capacity value for PTT was 3.571 mg/g, according to the result of R2 values, which showed a strong fit for the Langmuir model. The pseudo-second-order kinetic model provided the best explanation for the adsorption process. The results of the calculations of thermodynamic variables, such as ΔH, ΔG, and ΔS, at the ideal temperature of 40 0C were -6.022 KJ/mol, -2617.64 KJ/mol, and 3.495206 J/molK, respectively, confirming the adsorptive process's feasibility and exothermicity. Enthalpy was less than 84 KJ/mol. According to the results, PTT was an efficient adsorbent for eliminating CIP, and its adsorption capacity rose with temperature