Corrosion inhibition of mild-steel in sulphuric acid solution using expired Promethazine -Theoclate drug as inhibitor
Keywords:
Corrosion control, Mild steel, Sulphuric acid, Expired drug, InhibitorAbstract
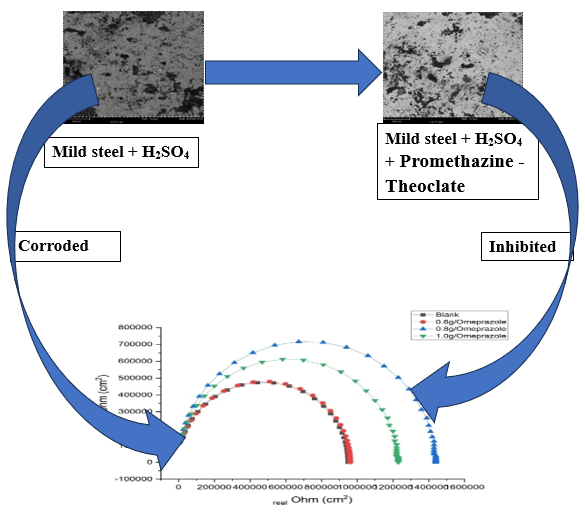
The purpose of this study is to investigate the effectiveness of expired promethazine-theoclate in sulphuric acid environment as mild-steel corrosion inhibitor. The drug was characterized for its functional groups and chemical constituents using infrared spectroscopy and gas chromatography methods. Experimental techniques and gravimetric methods were also employed. The inhibitory effects (thermodynamic and adsorption parameters) of the drug were assessed. Inhibition efficiencies were optimized and modeled using RSM and ANN models. The predominant functional groups were found to be O-H, CO-NH-CO stretches; =C-H stretching; N-H deformation, and contains 2, 4- di-tert-butylphenol, 1-Heptadecene, tridecane, Propyl 11-octadecenoate, and others. The heat of adsorption (Qads) results at various inhibitor concentrations were all negative with a value of -67151.6 J/mol with promethazine-theoclate concentration of 0.8 g/L. The Frumkin isotherm provided the best fit from the isotherm fittings since it gave the highest average R2. Gibb’s free energy of adsorption values of -10.23 kJ/mol and -10.29 kJ/mol at 313 K and 323 K, respectively, indicated that promethazine-theoclate molecules’ adsorption was physical and not chemisorption. Maximum efficiency of 92.89% was attained from the gravimetric method. The ANN gave better prediction of inhibition efficiency with higher value of R2 (0.9999) and lower values of RMSE (0.0180) and SEP (0.0230). RSM optimization gave an optimum efficiency of 92.39 %. The impedance method displayed a capacitive loop, signifying charge-transfer process, and polarization measurements indicated that the drug was a mixed-type inhibitor. Hence, promethazine-theoclate proved to be an excellent inhibitor for controlling mild steel corrosion in H2SO4 media.