Kinetics and Thermodynamics of Biolubricant Production from Lard Oil Using EG and TMP
Keywords:
biolubricant; lard base-oil; ethylene glycol (EG); trimethylolpropane (TMP); kinetic; thermodynamics; ethylene glycol biolubricant (LOBLEG); trimethylolpropane biolubricant (LOBLTMP)Abstract
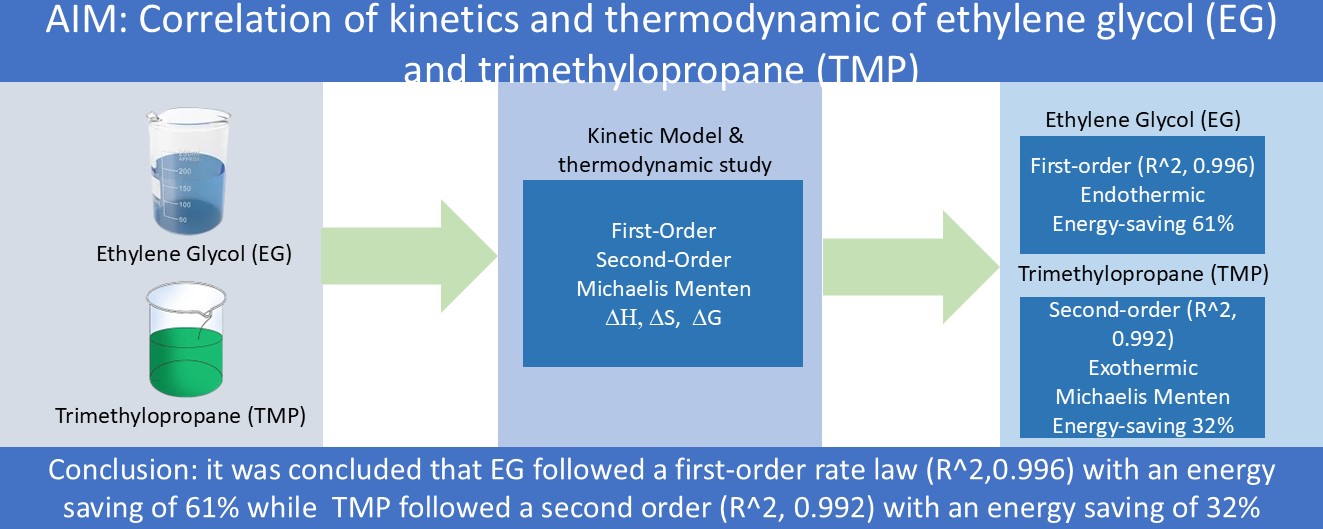
This study presents the kinetics and thermodynamics correlation of biolubricants derived from lard oil-based stock using ethylene glycol (EG) and trimethylolpropane (TMP). Kinetic data fitting for LOBLEG to both first and second-order kinetic rate laws showed that the R^2 value for the first-order model were higher than that for the second-order model. This implies that the kinetic data for LOBLEG follows a simpler kinetic behavior that can be easily modeled. In contrast, in LOBLTMP, the R^2 value of the second-order model was higher, indicating a better fit. In terms of thermodynamics, it was observed that the reaction process for LOBLEG was endothermic, with an ∆H value of 54.9 kJ/mol, while for LOBLTMP, it was exothermic with an ∆H value of -91.5 kJ/mol. Furthermore, the kinetic data for LOBLEG did not align with the Michaelis-Menten rate law, as indicated by an R^2 value of 0.81, in contrast to the R^2 value of 0.95 obtained for LOBLTMP which agrees closely with the kinetic reaction to produce LOBLTMP. These findings suggest that LOBLTMP exhibits a more temperature-driven reaction rate than LOBLEG, while the latter showed a higher energy-saving capacity due to lower activation energy. Thus, both routes offer means through which environmentally friendly lubricants can be produced and data provided here could be used to design reactors that can produce biolubricants.