Inhibition of Mild Steel Corrosion in Alkaline Media Using Imidazolium-Based Ionic Liquid
Keywords:
Corrosion inhibition, mild steel, ionic liquid, electrochemical analysis, alkaline mediaAbstract
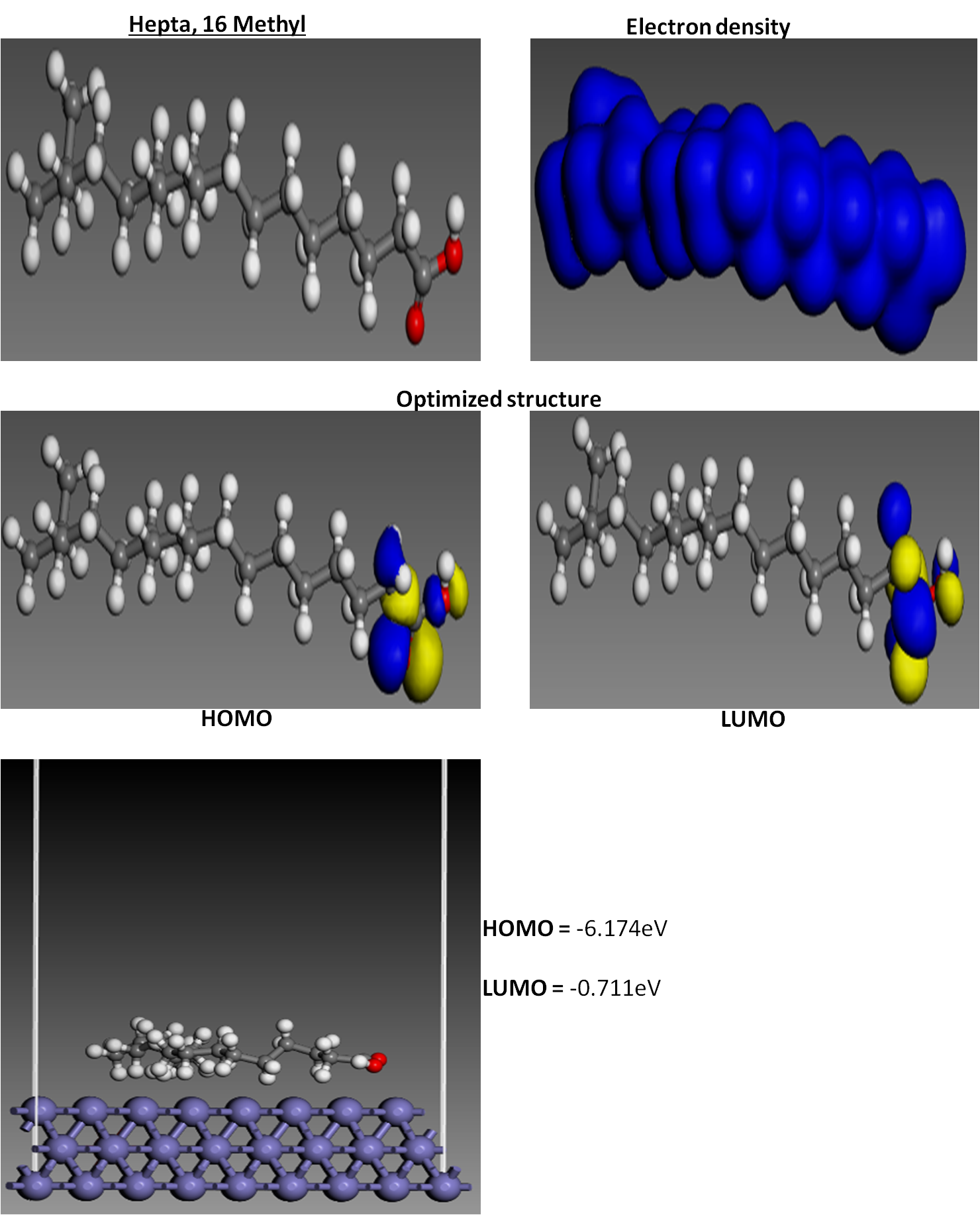
This study investigated the inhibition potentials of synthesized ionic liquid on mild steel corrosion in 1 M NaOH media, and the inhibition efficiency was evaluated. The synthesized ionic liquid achieved a maximum inhibition efficiency of 93.4% at 0.7 g/L inhibitor concentration and 313 K. Potentiodynamic polarization and electrochemical impedance spectroscopy revealed significant improvements in corrosion resistance, with a maximum charge transfer resistance of 136.5 Ω·cm². Adsorption studies indicated that the inhibitor adhered to the Langmuir isotherm (R² = 0.9961), and this finding implies monolayer adsorption of inhibitor molecules onto a homogeneous metal surface. The activation energy increased with inhibitor concentration, from 6.81 kJ/mol at 0.1 g/L to 78.49 kJ/mol at 0.9 g/L. The exothermic adsorption process was characterized by a maximum adsorption energy of -90.71 kJ/mol at 0.7 g/L. Scanning electron microscopy revealed that the steel surface smoothened upon the addition of the inhibitor. Density Functional Theory analysis further confirmed the strong adsorption and reactivity of the inhibitor molecules