Sustainability of Dye Effluent Using Adsorptive Properties of Awka Clay: Kinetics and Modeling (RSM, ANFIS, and ANN Analysis)
Keywords:
adsorption, isotherm, kinetic, modelling, thermodynamics, optimizationAbstract
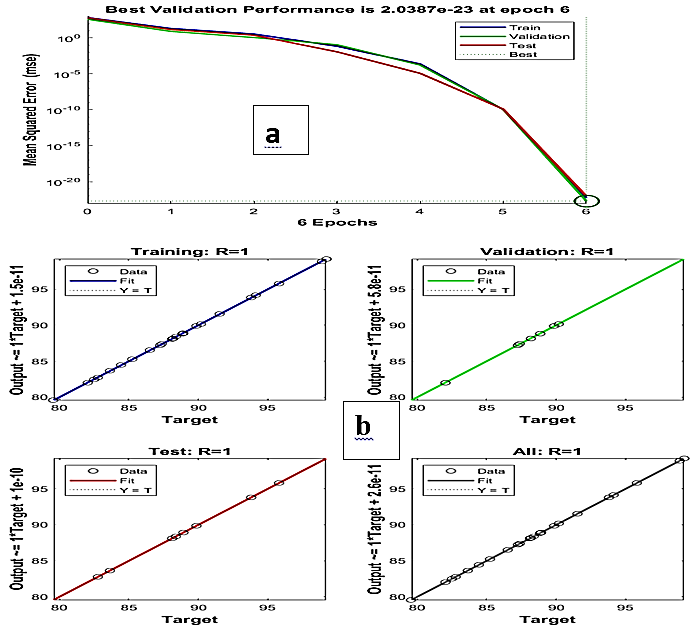
Azo dyes are toxic dyes of environmental concern due to their stable aromatic structure. The research work focused on the removal of CR pollutants from aqueous solution via utilizing the adsorptive qualities of acid-modified Awka clay (AMAC). The batch adsorption was conducted to investigate the process variables effect. The adsorption mechanism was investigated. The thermodynamic properties ΔS, ΔH, ΔG, and Ea were determined. The optimum CR removal was predicted using the ANN, ANFIS, and RSM models. The maximum dye removal of 99.99% was achieved at a temperature of 323 K, an adsorbent dosage of 1 g, a contact time of 150 min, an initial dye concentration of 100 mg/l, an adsorbent particle size of 75 μm, and pH 2. A maximum equilibrium adsorption capacity of 19.9999 mg/g was obtained. The adsorption mechanism result indicates that two or more steps influence the adsorption process. Thermodynamic results suggested an endothermic, favorable, spontaneous, and physical adsorption process. The RSM model, with an R² of unity, is statistically more significant than the ANN and ANFIS models. A maximum reusability capacity of 97.2% was achieved after three cycles. These obtained results confirm AMAC as a reliable adsorbent for CR removal from effluents.